Dyestuff industry experts
ITMA AISA 2024
- Home
- About
- Online Q & A
- Products
- Applications
- Solution
- Service
- Our Brand
- Info
- Contact
Views: 55 Author: Site Editor Publish Time: 2025-09-22 Origin: Site
The 1905 Nobel Prize in Chemistry was awarded to German chemist Adolf von Baeyer for his outstanding contributions to the synthesis of indigo and the hydrogenation of aromatic hydrocarbons.
Blue jeans, made from denim dyed with indigo, quickly became popular around the world in the late 1970s. Their popularity can be gauged from the dramatic increase in indigo production.
In the 1950s, annual indigo production in the United States was 15,000 tons.
By the mid-1960s, production had almost ceased.
By the late 1970s, production rebounded, with United Chemical Company alone producing 3,000 tons annually.
Since then, global production has increased at a rate exceeding 100%.
Indigo has been used for centuries. Ancient Egyptian mummies wore clothing dyed with indigo, and blue linen fabric unearthed from Mawangdui in China also contained indigo dye. Over 200 years ago, indigo was used on flags during the French Revolution and the American Revolutionary War. Its excellent colorfastness and lightfastness earned it the title “King of Dyes.”
Indigo is obtained from plants in the Indigofera group, such as:
Isatis indigotica
Polygonum tinctorium
Indigofera tinctoria
These plants were once widely cultivated in China and India. Their leaves, roots (Isatis root), and the processed precipitate (Indigo naturalis) were also used in medicine.

The dyeing process involves:
Chopping the plants into small pieces.
Soaking them in vats of water for fermentation.
The fermented liquid contains a leuco-solid (based on indole).
Fabric is soaked in this liquid and then air-dried.
Oxidation by air converts the leuco-solid into insoluble indigo, fixing it to the fabric.
Because this dyeing occurs in vats with minimal contact with air, indigo is classified as a vat dye.
Before Baeyer’s work, chemists had determined:
Empirical formula: C₈H₅NO
Molecular formula: C₁₆H₁₅N₂O₂
They also discovered its reactions:
With caustic potash → anthranilic acid
At high temperature → aniline
Oxidized → indigo red
But the structure could not be deduced at the time due to limited molecular theory.
In 1865, Kekulé proposed the benzene ring structure. In the same year, Baeyer began researching indigo.
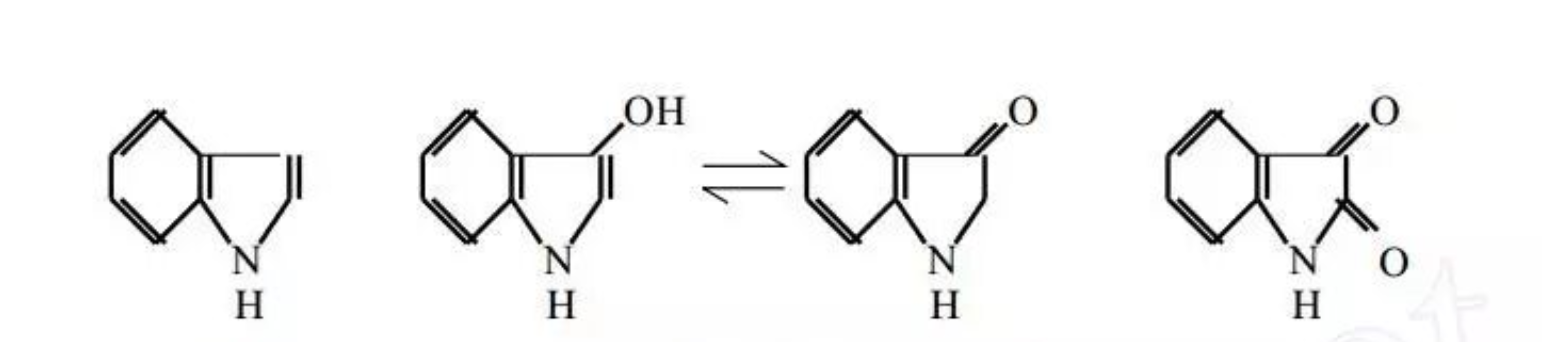
He proposed indole as the “parent” substance of indigo.
In 1866, Baeyer successfully produced indole by heating indigo derivatives with zinc powder, marking a breakthrough.
This zinc reduction method later helped Baeyer’s student C. Graebe determine the structure of alizarin and scale up its production (1871), stimulating the dye industry.
1870: Baeyer and students treated indigo with phosphorus trichloride and reduced it with zinc/HCl → produced artificial indigo.
1878: Indigo synthesized from phenylacetic acid.
1879–1880: Baeyer discovered new synthesis routes via o-nitrocinnamic acid and o-nitrophenylpropionic acid.
March 19, 1880: First patent for synthetic indigo.
December 1880: First scientific paper on indigo synthesis.
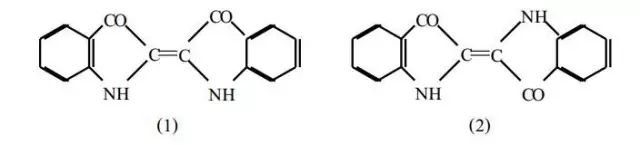
1883: Structural formula proposed (later refined in 1928 with X-ray diffraction).
Although Baeyer’s lab synthesis was not the industrial route, K. Heumann’s method (1890) using aniline and acetic acid became the foundation for large-scale production.
By the late 19th century:
Synthetic indigo factories replaced natural indigo farms worldwide.
Indigo and alizarin industrialization spurred the rise of Germany’s organic chemical industry.
Many leaders in this new industry were trained under Baeyer, cementing his role as both a pioneer chemist and influential educator.
The story of indigo is a journey from ancient natural dyeing traditions to modern industrial chemistry. Once extracted painstakingly from plants, indigo became a symbol of cultural identity, from ancient textiles to revolutionary flags and, later, global fashion through denim.
Adolf von Baeyer’s systematic research not only unveiled the structure and synthesis of indigo but also laid the foundation for the modern organic chemical industry. His contributions, crowned with the 1905 Nobel Prize, bridged the gap between traditional craftsmanship and industrial-scale dye production.
Today, indigo remains one of the most iconic dyes in the world—a symbol of both heritage and scientific progress, carrying forward the legacy of innovation and discovery that transformed chemistry and global industry.
Contact tiankun chemical to know more details about indigo blue by info@tiankunchemical.com